*Based on IQVIA prescription data collected from December 2011 to August 2023.
Study Designs
COMBI-v
COMBI-v was a phase 3, multicenter, open-label, randomized (1:1), active-controlled trial of 704 patients with unresectable (stage IIIC) or metastatic (stage IV) BRAF V600E/K mutation–positive cutaneous melanoma. Patients were randomized to receive TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily (n=352) or single-agent vemurafenib 960 mg twice daily (n=352). Treatment was continued until disease progression or unacceptable toxicity. The primary end point was OS. Secondary end points included progression-free survival (PFS), overall response rate (ORR), duration of response (DOR), and safety.1,2,5
COMBI-d
COMBI-d was a phase 3, multicenter, double-blind, randomized (1:1), active-controlled trial of 423 patients with unresectable (stage IIIC) or metastatic (stage IV) BRAF V600E/K mutation–positive cutaneous melanoma. Patients were randomized to receive TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily (n=211) or TAFINLAR 150 mg twice daily + placebo (n=212). Treatment was continued until disease progression or unacceptable toxicity. The primary end point was PFS. Secondary end points included OS, DOR, ORR, and safety. At the time of the primary analysis (August 2013), patients taking TAFINLAR + MEKINIST were progression free for a median of 9.3 months (95% CI, 7.7-11.1) vs 8.8 months (95% CI, 5.9-10.9) with TAFINLAR + matching placebo (HR, 0.75; 95% CI, 0.57-0.99; P=.035). In the interim analysis of COMBI-d, median OS was 25.1 months (95% CI, 19.2-NR) in the TAFINLAR + MEKINIST group and 18.7 months (95% CI, 15.2-23.1) in the TAFINLAR + placebo group (HR, 0.71; 95% CI, 0.55-0.92; P=.01).1,2,7
Pooled COMBI-d/v
Pooled analysis of COMBI-d and COMBI-v consisted of 563 first-line patients with BRAF V600E/K mutation–positive unresectable or metastatic melanoma. All patients in the analysis received TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily in either COMBI-d (data cutoff, December 10, 2018) or COMBI-v (data cutoff, October 8, 2018). Median follow-up was 22 months (range, 0-76).4,8
BRF113220
BRF113220 was an open-label phase 1 and 2 study of patients with unresectable stage IIIC or IV BRAF V600E/K mutation–positive melanoma. This study had 4 parts (A, B, C, D). Analysis only includes patients enrolled in Part C. Patients were randomly assigned 1:1:1 to receive either TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily, TAFINLAR 150 mg twice daily + MEKINIST 1 mg once daily, or TAFINLAR 150 mg monotherapy twice daily. Patients who progressed in the TAFINLAR monotherapy arm were allowed to cross over to the TAFINLAR 150-mg twice daily + MEKINIST 2-mg once daily arm. Efficacy and safety were analyzed at 4 and 5 years. End points included treatment response, DOR, PFS, OS, and safety.9
COMBI-MB
COMBI-MB was a phase 2, nonrandomized, open-label, multicenter, 4-cohort trial of 121 patients with BRAF V600E/K mutation–positive melanoma and at least 1 measurable intracranial lesion. Patients received TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily. Treatment was continued until disease progression or unacceptable toxicity. The primary end point was intracranial response rate, defined as the percentage of patients with a confirmed intracranial response per Response Evaluation Criteria In Solid Tumors v1.1, modified to allow up to 5 intracranial target lesions at least 5 mm in diameter, as assessed by independent review.1,2,10
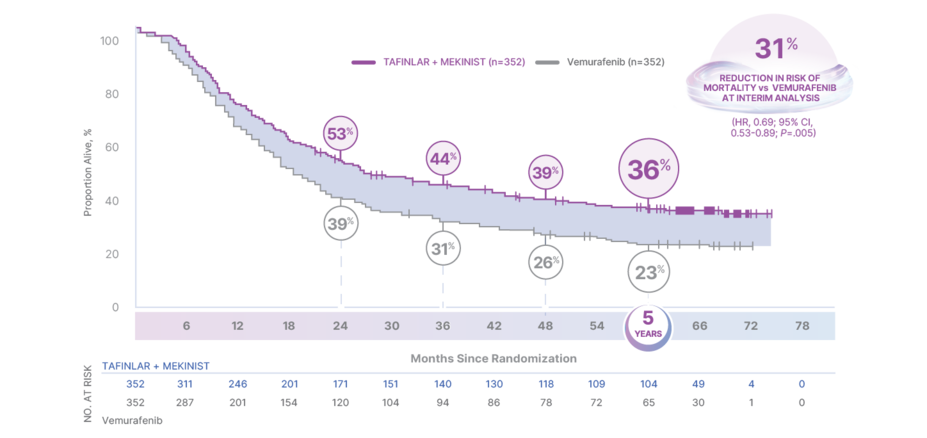
5-Year Overall Survival and Progression-Free Survival—COMBI-v
In COMBI-v—For the first-line treatment of patients with BRAF V600E/K unresectable or metastatic melanoma
THE STRENGTH OF DURABLE SURVIVAL4
36% OF PATIENTS WERE ALIVE AT 5 YEARS4
OS in COMBI-v4,5
HR, hazard ratio; OS, overall survival.
At the interim analysis (April 2014), the median OS was NR for patients taking TAFINLAR + MEKINIST (95% CI, 18.3-NR) vs 17.2 months for vemurafenib (95% CI, 16.4-NR)5,6
Risk of mortality reduced by 31% (HR, 0.69; 95% CI, 0.53-0.89; P=.005)
Crossover was permitted after an interim OS analysis demonstrating significant benefit in COMBI-v4,†
Results at 24, 36, 48, and 60 months were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
OS IS ONE OF THE MOST IMPORTANT MEASURES OF EFFICACY IN METASTATIC MELANOMA; TAFINLAR + MEKINIST has high OS rates at 4 and 5 years in a phase 3 trial4
†COMBI-v was stopped early by the Independent Data Monitoring Committee after the preplanned interim analysis (222 events; 32%) conducted in April 2014 showed OS results that crossed the prespecified efficacy stopping boundary (P<.0214), at which point the interim analysis became final. Patients will continue to be followed until death, withdrawal of consent, loss to follow-up, or have had at least 5 years of follow-up.5,6,11
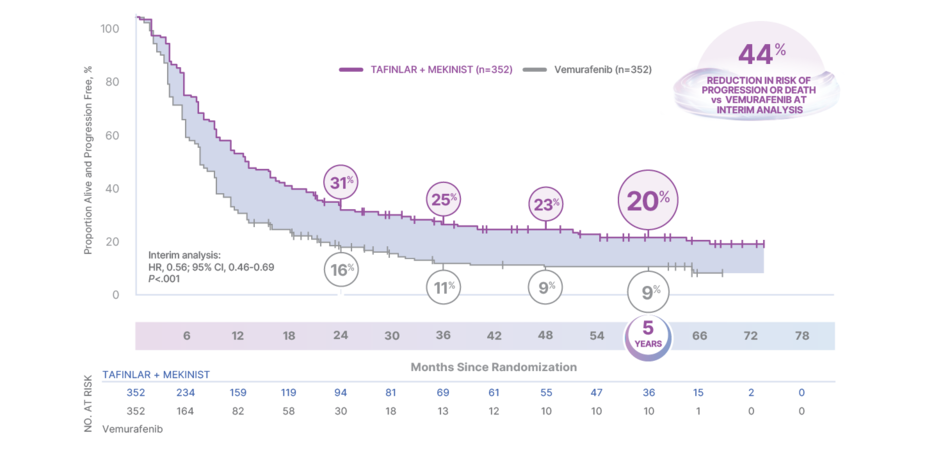
THE STRENGTH TO DELAY PROGRESSION5
20% OF PATIENTS LIVING PROGRESSION FREE AT 5 YEARS4
PFS analysis of the intention-to-treat population in COMBI-v4,5
HR, hazard ratio; PFS, progression-free survival.
In the interim analysis, median PFS was 11.4 months (95% CI, 9.9-14.9) for TAFINLAR + MEKINIST and 7.3 months (95% CI, 5.8-7.8) for vemurafenib (HR, 0.56; 95% CI, 0.46-0.69; P<.001)1
Results at 24, 36, 48, and 60 months were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
TAFINLAR + MEKINIST CONTINUES TO BE THE MOST PRESCRIBED COMBINATION TARGETED THERAPY for patients with BRAF+ melanoma WORLDWIDE, as of December 20213,‡
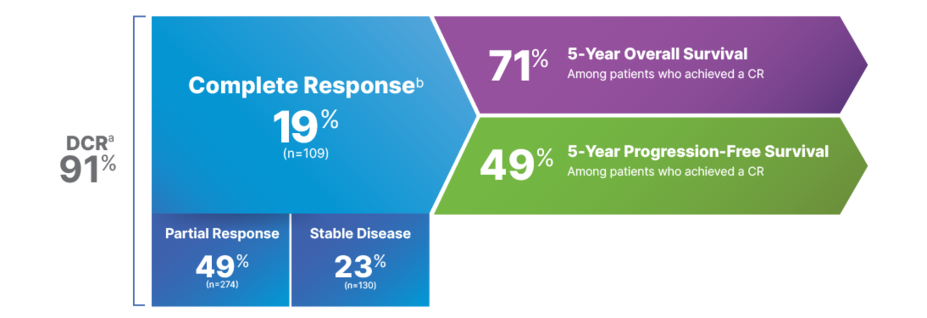
5-Year Response Rates–Pooled COMBI-d/v Analysis
In the pooled analysis of COMBI-d and COMBI-v—For the first-line treatment of patients with BRAF V600E/K unresectable or metastatic melanoma
DEPTH OF RESPONSE THAT LASTS4,8
Response across pooled COMBI-d/v data4,8
CR, complete response; DCR, disease control rate; ORR, overall response rate; SD, stable disease.
aDCR is defined as CR + partial response + SD; SD is not a component of ORR and can reflect the natural progression of disease rather than a direct therapeutic effect.
bTwo patients are excluded from this analysis because they had no measurable disease at baseline.4
In the primary analysis for COMBI-d, complete response (CR) for patients taking TAFINLAR + MEKINIST was 10%, partial response (PR) was 56%, and stable disease (SD) was 26%, for a total disease control rate (DCR) of 92%. For patients taking TAFINLAR monotherapy, CR was 9%, PR was 43%, and SD was 33%, for a total DCR of 84%7
In the interim analysis for COMBI-v, CR for patients taking TAFINLAR + MEKINIST was 13%, PR was 51%, and SD was 26%, for a total DCR of 91%. For patients taking vemurafenib, CR was 8%, PR was 44%, and SD was 30%, for a total DCR of 82%5
Results at 5 years were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
EFFICACY PROVEN IN A LARGER PHASE 3 TRIAL PATIENT POPULATION THAN ANY OTHER COMBINATION TARGETED THERAPY1,2,12-18
Time to Response
In BRF113220—For the first-line treatment of patients with BRAF V600E/K unresectable or metastatic melanoma
A STRONG START: GIVE YOUR PATIENTS A CHANCE FOR A RAPID RESPONSE
In a phase 1 and 2, open-label study of TAFINLAR + MEKINIST in patients with unresectable stage IIIC or IV BRAF V600E/K–mutant metastatic melanoma9
The primary end point was PFS; PFS in the TAFINLAR + MEKINIST group at 48 and 60 months was 13% (95% CI, 5%-25%) vs 3% (95% CI, 0%-11%) for TAFINLAR alone (HR, 0.44; 95% CI, 0.28-0.67)9
AMONG PATIENTS WHO RESPONDED, 50% HAD ALREADY ACHIEVED A RESPONSE BY 1.8 MONTHS9
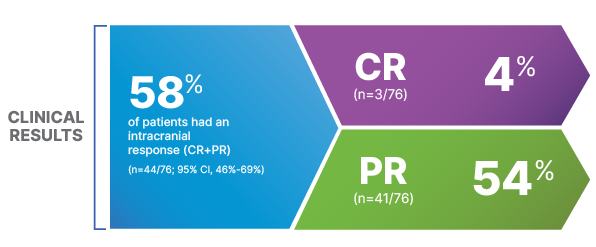
Efficacy in Patients With Brain Metastases
In COMBI-MB–For the first-line treatment of patients with BRAF V600E/K unresectable or metastatic melanoma
THE STRENGTH OF AN INTRACRANIAL RESPONSE
MORE THAN HALF OF PATIENTS WITH BRAIN METASTASES SHOWED AN INTRACRANIAL RESPONSE WITH TAFINLAR + MEKINIST10
COMBI-MB: Clinical Results (Cohort A)10
CR, complete response; PR, partial response.
50% of patients in COMBI-MB had an intracranial response (95% CI, 41%-60%), with a complete response rate of 4.1% and a partial response rate of 46%1,2