Testing for BRAF V600E Mutation in Metastatic NSCLC
TARGETED THERAPY IS THE GUIDELINE-RECOMMENDED FIRST-LINE OPTION FOR PATIENTS WITH BRAF V600E METASTATIC NSCLC
Why test for BRAF V600E
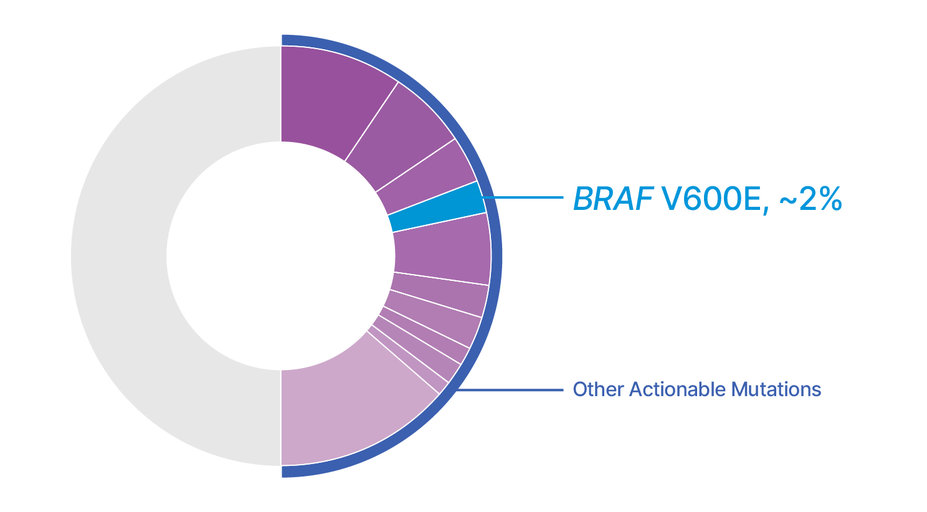
Nearly 1 in 2 patients with metastatic NSCLC may have an actionable driver mutation that can be treated with a targeted therapy.1-10,*
A patient with BRAF V600E could have any number of characteristics. Testing patients using comprehensive genomic profiling can help determine whether TAFINLAR + MEKINIST may be the appropriate treatment option.
According to the National Comprehensive Cancer Network® (NCCN®), although PD-L1 expression can be elevated in patients with an oncogenic driver, targeted therapy for the oncogenic driver should take precedence over treatment with an immune checkpoint inhibitor14,‡
TAFINLAR + MEKINIST does not have any head-to-head data against an immunotherapy, and no sequencing studies have been conducted. PD-L1 expression was not the basis for inclusion or exclusion for the clinical trials with TAFINLAR + MEKINIST.
1. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321-1328. doi:10.1093/annonc/mdz167 2. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi:10.1056/NEJMoa1713137 3. Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naive patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138-1145. doi:10.1016/j.jtho.2018.03.035 4. Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14(7):1233-1243. doi:10.1016/j.jtho.2019.03.007 5. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118-1133. doi:10.1158/2159-8290.CD-16-0596 6. Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254-266. doi:10.6004/jnccn.2021.0013
When to test for BRAF V600E
To inform up-front treatment planning, test every patient at diagnosis using comprehensive genomic profiling.
How to test for BRAF V600E
FoundationOne®CDx is FDA approved to help identify BRAF V600E mutation in metastatic NSCLC.
NSCLC, non-small cell lung cancer.
The NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
*Prevalence rates are in accordance with those from The Cancer Genome Atlas (TCGA) Research Network, a joint effort between the National Cancer Institute and the National Human Genome Research Institute. To access the latest TCGA data, please visit: www.cancer.gov/ccg/research/genome-sequencing/tcga.
†This calculation is based on a 2% prevalence rate and metastatic NSCLC–specific incidence and recurrence data from Kantar Health.
FoundationOne®CDx is a qualitative next-generation sequencing based in vitro diagnostic test for advanced cancer patients with solid tumors and is for prescription use only. The test analyzes 324 genes as well as genomic signatures including microsatellite instability (MSI) and tumor mutational burden (TMB) and is a companion diagnostic to identify patients who may benefit from treatment with specific therapies in accordance with the approved therapeutic product labeling. Additional genomic findings may be reported and are not prescriptive or conclusive for labeled use of any specific therapeutic product. Use of the test does not guarantee a patient will be matched to a treatment. A negative result does not rule out the presence of an alteration. Some patients may require a biopsy. For the complete label, including companion diagnostic indications and important risk information, please visit www.F1CDxLabel.com ↗.
The FoundationOne®CDx is a registered trademark of Foundation Medicine, Inc.