Dosing & Administration
Adult Dosing and Dose Reductions
Adult dosing:
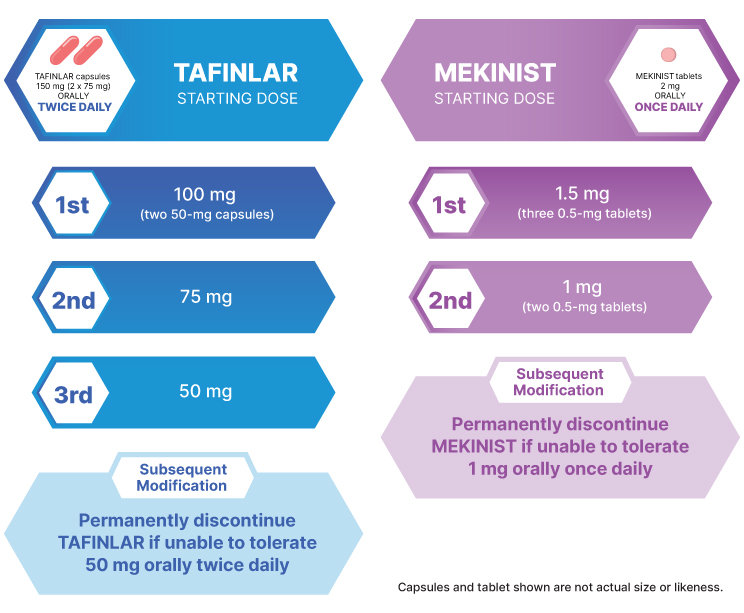
The recommended dosage for TAFINLAR in adult patients is 150 mg (two 75-mg capsules) taken orally twice daily1
The recommended dosage for MEKINIST in adult patients is 2 mg taken orally once daily2
TAFINLAR and MEKINIST should be taken at the same time each day, 1 hour prior or 2 hours after a meal1,2
TAFINLAR doses should be taken 12 hours apart. Missed doses of TAFINLAR should not be taken within 6 hours of the next dose1
MEKINIST doses should be taken 24 hours apart. Missed doses of MEKINIST should not be taken within 12 hours of the next dose2
TAFINLAR and MEKINIST can be administered in capsule and tablet form, respectively1,2
Treatment for patients with BRAF V600E+ solid tumors is recommended until disease progression or unacceptable toxicity1,2
Adult dose reductions1,2
The overall management of certain adverse reactions may require treatment interruption, dose reduction, or treatment discontinuation, depending on severity1,2
Pediatric Dosing and Dose Reductions
For the first time, TAFINLAR + MEKINIST is available in 2 forms for oral dosing1,2
Capsule and tablet pediatric dosing:
The recommended dosage for TAFINLAR (capsules) and MEKINIST (tablets) in pediatric patients is based on body weight, beginning at 26 kg. (Note: This applies to capsule and tablet dosing only.) A recommended dose for TAFINLAR + MEKINIST has not been established in patients who weigh less than 26 kg1,2
Weight-based oral dosing for TAFINLAR capsules and MEKINIST tablets1,2
TAFINLAR | MEKINIST | ||
Body Weight | Recommended Pediatric Dose (Capsules) | Body Weight | Recommended Pediatric Dose (Tablets) |
26 to 37 kg | 75 mg (one 75-mg capsule) orally BID | 26 to 37 kg | 1 mg (two 0.5-mg tablets) orally QD |
38 to 50 kg | 100 mg (two 50-mg capsules) orally BID | 38 to 50 kg | 1.5 mg (three 0.5-mg tablets) orally QD |
≥51 kg | 150 mg (two 75-mg capsules) orally BID | ≥51 kg | 2 mg (one 2-mg tablet) orally QD |
BID, twice daily; QD, once daily.
Treatment is recommended until disease progression or unacceptable toxicity1,2
The number of TAFINLAR capsules and MEKINIST tablets included in the chart above are an example. TAFINLAR and MEKINIST are also available in other strengths. TAFINLAR is available as 50-mg and 75-mg capsules, and MEKINIST is available as 0.5-mg and 2-mg tablets1,2
TAFINLAR and MEKINIST should be taken at the same time each day, 1 hour prior or 2 hours after a meal1,2
TAFINLAR doses should be taken 12 hours apart. Missed doses of TAFINLAR should not be taken within 6 hours of the next dose1
MEKINIST doses should be taken 24 hours apart. Missed doses of MEKINIST should not be taken within 12 hours of the next dose2
TAFINLAR and MEKINIST can be administered in capsule and tablet form, respectively1,2
Liquid formulation dosing:
Weight-based dosing for TAFINLAR tablets for oral suspension and MEKINIST for oral solution1,2
TAFINLAR Total Daily Dose | MEKINIST Total Daily Dose | ||
Body Weight | Recommended Dosage (# of 10-mg Tablets of Oral Suspension BID) | Body Weight | Recommended Dosage (Total Volume of Oral Solution QD) |
8 to 9 kg | 20 mg (2 tablets) | 8 kg | 6 mL (0.3 mg) |
9 kg | 7 mL (0.35 mg) | ||
10 to 13 kg | 30 mg (3 tablets) | 10 kg | 7 mL (0.35 mg) |
11 kg | 8 mL (0.4 mg) | ||
12 to 13 kg | 9 mL (0.45 mg) | ||
14 to 17 kg | 40 mg (4 tablets) | 14 to 17 kg | 11 mL (0.55 mg) |
18 to 21 kg | 50 mg (5 tablets) | 18 to 21 kg | 14 mL (0.7 mg) |
22 to 25 kg | 60 mg (6 tablets) | 22 to 25 kg | 17 mL (0.85 mg) |
26 to 29 kg | 70 mg (7 tablets) | 26 to 29 kg | 18 mL (0.9 mg) |
30 to 33 kg | 80 mg (8 tablets) | 30 to 33 kg | 20 mL (1 mg) |
34 to 37 kg | 90 mg (9 tablets) | 34 to 37 kg | 23 mL (1.15 mg) |
38 to 41 kg | 100 mg (10 tablets) | 38 to 41 kg | 25 mL (1.25 mg) |
42 to 45 kg | 110 mg (11 tablets) | 42 to 45 kg | 28 mL (1.4 mg) |
46 to 50 kg | 130 mg (13 tablets) | 46 to 50 kg | 32 mL (1.6 mg) |
≥51 kg | 150 mg (15 tablets) | ≥51 kg | 40 mL (2 mg) |
BID, twice daily; QD, once daily.
Treatment is recommended until disease progression or unacceptable toxicity1,2
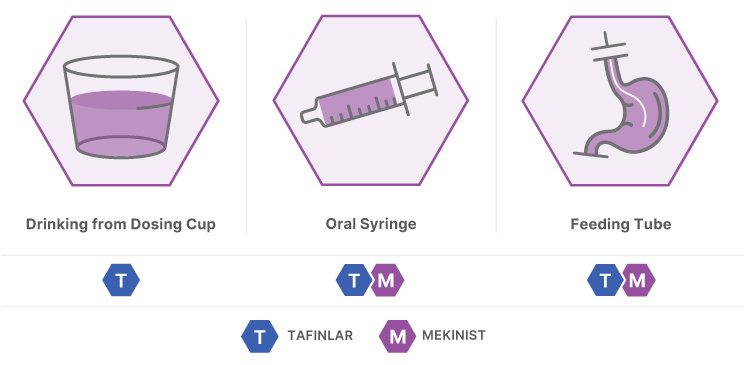
TAFINLAR + MEKINIST can be administered as a liquid formulation for patients who may have difficulty swallowing or are unable to swallow3
TAFINLAR oral suspension is prepared by dissolving TAFINLAR tablets in 5 to 10 mL of water. MEKINIST is provided as an oral solution by your patient’s pharmacy1,2
TAFINLAR + MEKINIST liquid formulation can be administered in multiple ways1,2:
Pediatric dose reductions
The overall management of certain adverse reactions may require treatment interruption, dose reduction, or treatment discontinuation, depending on severity1,2
Dose reductions for TAFINLAR capsules and MEKINIST tablets1,2
TAFINLAR Recommended Pediatric Dose Reductions (Capsules) | MEKINIST Recommended Pediatric Dose Reductions (Tablets) |
Body Weight: 26 to 37 kg | |
Recommended dose: 75 mg (one 75-mg capsule) orally BID
| Recommended dose: 1 mg (two 0.5-mg tablets) orally QD
|
Body Weight: 38 to 50 kg | |
Recommended dose: 100 mg (two 50-mg capsules) orally BID
| Recommended dose: 1.5 mg (three 0.5-mg tablets) orally QD
|
Body Weight: ≥51 kg | |
Recommended dose: 150 mg (two 75-mg capsules) orally BID
| Recommended dose: 2 mg (one 2-mg tablet) orally QD
|
BID, twice daily; QD, once daily.
Dose reductions for TAFINLAR + MEKINIST liquid formulation1,2
TAFINLAR Total Daily Dose (Oral Suspension) | MEKINIST Total Daily Dose (Oral Solution) | |||||||
Body Weight | Recommended Dosage BID | Dose Reductiona | Body Weight | Recommended Dosage QD | Dose Reduction | |||
1st | 2nd | 3rd | 1st | 2nd | ||||
8 to 9 kg | 20 mg | 1 | 0 | 0 | 8 kg | 6 mL (0.3 mg) | 5 mL | 3 mL |
9 kg | 7 mL (0.35 mg) | 5 mL | 4 mL | |||||
10 to 13 kg | 30 mg | 2 | 1 | 0 | 10 kg | 7 mL (0.35 mg) | 5 mL | 4 mL |
11 kg | 8 mL (0.4 mg) | 6 mL | 4 mL | |||||
12 to 13 kg | 9 mL (0.45 mg) | 7 mL | 5 mL | |||||
14 to 17 kg | 40 mg | 3 | 2 | 1 | 14 to 17 kg | 11 mL (0.55 mg) | 8 mL | 6 mL |
18 to 21 kg | 50 mg | 3 | 2 | 1 | 18 to 21 kg | 14 mL (0.7 mg) | 11 mL | 7 mL |
22 to 25 kg | 60 mg | 4 | 3 | 2 | 22 to 25 kg | 17 mL (0.85 mg) | 13 mL | 9 mL |
26 to 29 kg | 70 mg | 5 | 4 | 2 | 26 to 29 kg | 18 mL (0.9 mg) | 14 mL | 9 mL |
30 to 33 kg | 80 mg | 5 | 4 | 3 | 30 to 33 kg | 20 mL (1 mg) | 15 mL | 9 mL |
34 to 37 kg | 90 mg | 6 | 5 | 3 | 34 to 37 kg | 23 mL (1.15 mg) | 17 mL | 12 mL |
38 to 41 kg | 100 mg | 7 | 5 | 3 | 38 to 41 kg | 25 mL (1.25 mg) | 19 mL | 13 mL |
42 to 45 kg | 110 mg | 7 | 6 | 4 | 42 to 45 kg | 28 mL (1.4 mg) | 21 mL | 14 mL |
46 to 50 kg | 130 mg | 9 | 7 | 4 | 46 to 50 kg | 32 mL (1.6 mg) | 24 mL | 16 mL |
≥51 kg | 150 mg | 10 | 8 | 5 | ≥51 kg | 40 mL (2 mg) | 30 mL | 20 mL |
Permanently discontinue MEKINIST for oral solution if unable to tolerate a maximum of 2 dose reductions.2
BID, twice daily; QD, once daily.
aNumber of 10-mg tablets for oral suspension BID.
Recommended Dose Modifications
Severity of Adverse Reactiona | TAFINLAR1,b | MEKINIST2,c |
Hemorrhage | ||
| See Other Adverse Reactions (Including Hemorrhage for TAFINLAR) | Withhold MEKINIST
|
| See Other Adverse Reactions (Including Hemorrhage for TAFINLAR) | Permanently discontinue MEKINIST |
New Primary Malignancies | ||
| Permanently discontinue TAFINLAR | No dose modifications recommended for MEKINIST |
Venous Thromboembolism | ||
| No dose modifications recommended for TAFINLAR | Withhold MEKINIST for up to 3 weeks
|
| No dose modifications recommended for TAFINLAR | Permanently discontinue MEKINIST |
Cardiomyopathy | ||
| No dose modifications recommended for TAFINLAR | Withhold MEKINIST for up to 4 weeks
|
| Withhold TAFINLAR until LVEF improves to at least the institutional LLN and absolute decrease to less than or equal to 10% compared to baseline, then resume at same dose | Permanently discontinue MEKINIST |
Ocular Toxicities | ||
| No dose modifications recommended for TAFINLAR | Withhold MEKINIST for up to 3 weeks
|
| No dose modifications recommended for TAFINLAR | Permanently discontinue MEKINIST |
| Withhold TAFINLAR for up to 6 weeks
| No dose modifications recommended for MEKINIST |
Pulmonary | ||
| No dose modifications recommended for TAFINLAR | Permanently discontinue MEKINIST |
Febrile Reactions | ||
| Withhold TAFINLAR until fever resolves, then resume at same or lower dose | Withhold MEKINIST until fever resolves, then resume MEKINIST at same or lower dose |
|
Or
|
Or
|
Skin Toxicities | ||
| Withhold TAFINLAR for up to 3 weeks
| Withhold MEKINIST for up to 3 weeks
|
| Permanently discontinue TAFINLAR | Permanently discontinue MEKINIST |
Other Adverse Reactions (Including Hemorrhage for TAFINLAR) | ||
| Withhold TAFINLAR
| Withhold MEKINIST
|
|
Or
|
Or
|
| Permanently discontinue TAFINLAR | Permanently discontinue MEKINIST |
DVT, deep venous thrombosis; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; PE, pulmonary embolism.
aNational Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
bDose modifications are not recommended for TAFINLAR when administered with MEKINIST for the following adverse reactions of MEKINIST: retinal vein occlusion, retinal pigment epithelial detachment, interstitial lung disease/pneumonitis, and uncomplicated venous thromboembolism. Dose modification of TAFINLAR is not required for new primary cutaneous malignancies.
cDose modifications are not recommended for MEKINIST when administered with TAFINLAR for the following adverse reactions of TAFINLAR: noncutaneous malignancies and uveitis. Dose modification of MEKINIST is not required for new primary cutaneous malignancies.
Storage and Handling
Storage for capsules and tablets:
TAFINLAR capsules should be stored at room temperature between 68 °F to 77 °F (20 °C–25 °C); excursions permitted between 59 °F and 86 °F (15 °C and 30 °C); store and dispense in the original bottle with the desiccant1
MEKINIST tablets should be stored at room temperature between 68 °F to 77 °F (20 °C–25 °C); dispense in original bottle; do not remove desiccant; protect from moisture and light; do not place medication in pill boxes2
Storage for TAFINLAR tablets for oral suspension and MEKINIST for oral solution:
TAFINLAR should be stored at 68 °F to 77 °F (20 °C–25 °C); excursions permitted between 59 °F and 86 °F (15 °C and 30 °C); store and dispense in the original bottle with the desiccant1
MEKINIST should be stored refrigerated at 36 °F to 46 °F (2 °C–8 °C); store in the original carton to protect from light and moisture. After reconstitution, store in the original bottle below 77 °F (25 °C) and do not freeze2
For complete dosage and storage information, please see the full Prescribing Information for the appropriate medications.