Study Design
For the adjuvant treatment of patients with BRAF V600E/K melanoma and involvement of lymph node(s), following complete resection
COMBI-AD STUDY DESIGN1,2,6
12 months of treatmenta
aRadiological tumor assessment was conducted every 3 months for the first 2 years and every 6 months thereafter, until first relapse was observed. The median duration of follow-up (time from randomization to last contact or death) was 2.8 years for primary analysis. bEnrollment required complete resection of melanoma with complete lymphadenectomy within 12 weeks prior to randomization. All patients had no prior systemic anticancer treatment, including radiotherapy. Patients with a history of prior malignancy, if disease-free for at least 5 years, were eligible. cDefined as the time from randomization to disease recurrence, new primary melanoma, or death from any cause.
KEY ELIGIBILITY CRITERIA1,2,6
Completely resected, high-risk stage IIIA (lymph-node metastasis >1 mm), IIIB, or IIIC cutaneous melanoma
BRAF V600E/K mutations
Surgically free of disease ≤12 weeks before randomization
Eastern Cooperative Oncology Group performance status of 0 or 1
No prior radiotherapy or anticancer systemic therapy
COMBI-AD included patients with substages A-C of stage III disease prior to resection7
19% of these patients had micrometastasis and no primary tumor ulceration (n=165/870 with no ulceration and micrometastasis; 5-year update)
Follow-up until end of study8
Patients were followed for disease recurrence until the first recurrence and thereafter for survival
The study will be considered complete and final overall survival (OS) analysis will occur when ≈70% of randomized patients have died or are lost to follow-up
10-Year Relapse-Free Survival
With data after 10 years of follow-up,
DEFY EXPECTATIONS FOR RELAPSE-FREE SURVIVAL1-3
TAFINLAR + MEKINIST DELIVERED AN EARLY RFS BENEFIT WITH THE SEPARATION OF CURVE THAT WAS SUSTAINED AT 10 YEARS vs PLACEBO1-3
RFS in COMBI-ADa
aData as of July 31, 2023. First tumor assessment was performed at 3 months.1-3
bHR was estimated using a Pike estimator.3
In the primary analysis, the median RFS was not reached (NR) in the TAFINLAR + MEKINIST group (95% CI, 44.5-not estimable) and 16.6 months in the placebo group (95% CI, 12.7-22.1); (HR, 0.47; 95% CI, 0.39-0.58; P<.0001)1,2
At the 10-year follow-up, median RFS was 93.1 months (95% CI, 47.9-NR) vs 16.6 months (95% CI, 12.7-22.1) for placebo (HR, 0.52; 95% CI, 0.43-0.63)3
Median duration of follow-up for the final analysis was 100 months for the TAFINLAR + MEKINIST treatment arm and 82.5 months for the placebo arm9
Results at 1 year, 5 years, and 10 years were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
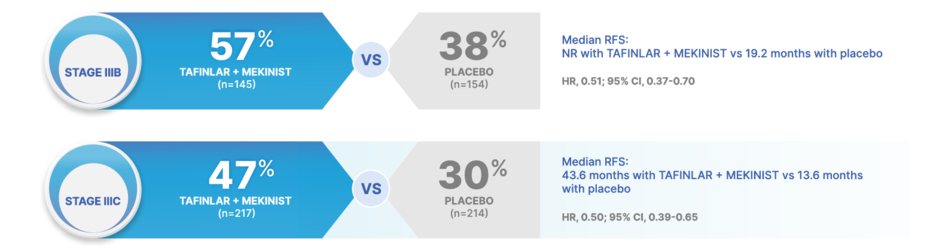
RAISE EXPECTATIONS FOR RELAPSE-FREE SURVIVAL ACROSS ALL OF STAGE III MELANOMA10
At 5 years, TAFINLAR + MEKINIST achieved higher RFS rates across stage III substages of melanoma (per AJCC 8 criteria) vs placebo
50% REDUCTION IN THE RISK OF RELAPSE OR DEATH WITH TAFINLAR + MEKINIST vs placebo in patients with stage IIIC disease10
Stage IIIA: HR, 0.83 (95% CI, 0.36-1.91) for TAFINLAR + MEKINIST (n=50) vs placebo (n=39)10
Stage IIID: HR, 0.34 (95% CI, 0.14-0.79) for TAFINLAR + MEKINIST (n=22) vs placebo (n=17)10
Results at 60 months were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
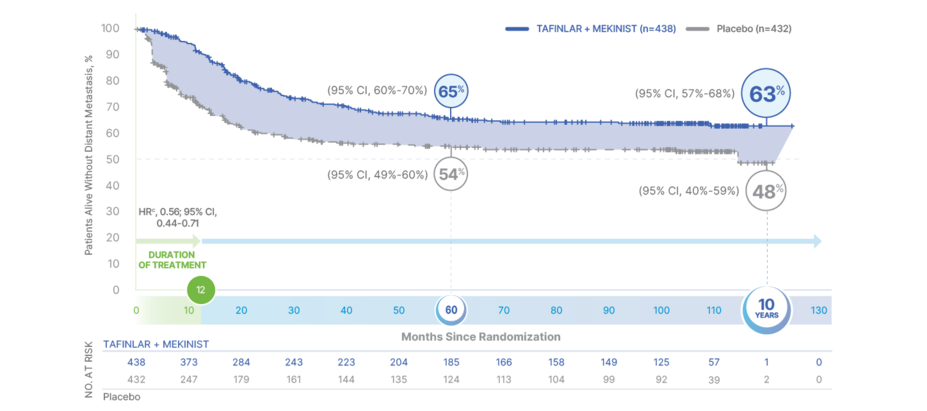
10-Year Distant Metastasis-Free Survival
With 10 years of follow-up,
DEFY EXPECTATIONS FOR DISTANT METASTASIS-FREE SURVIVAL3
TAFINLAR + MEKINIST REDUCED THE RISK OF DEVELOPING METASTATIC DISEASE BY 44% vs PLACEBO AT 10 YEARS3
DMFSa 10 years after surgery in patients treated with TAFINLAR + MEKINIST vs placebo3,b
aDMFS is defined as the time from randomization to the date of first distant metastasis or date of death, whichever occurred first.7
bData as of July 31, 2023.3
cHR was estimated using a Pike estimator.3
Distant metastasis-free survival (DMFS) favored the treatment arm vs placebo at the primary and final analyses. Median DMFS was NR in the TAFINLAR + MEKINIST arm vs 114.6 months (95% CI, 49.8-NR) in the placebo arm3,6
Primary analysis (median follow-up 2.8 years): HR, 0.51 (95% CI, 0.40-0.65)6
Final analysis (median follow-up 8.3 years): HR, 0.56 (95% CI, 0.44-0.71)3,9
Results at 5 years and 10 years were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
ABOUT DMFS: For local recurrence, surgery is still an option.11 Distant recurrence, however, signals progression to metastatic disease and is associated with lower survival.12 With TAFINLAR + MEKINIST, 63% of patients were free of distant metastases and alive at 10 years.3
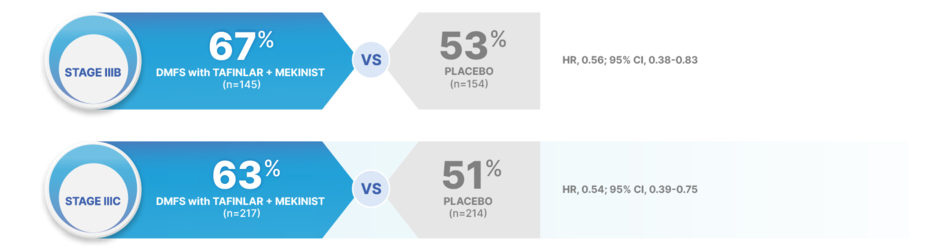
RAISE EXPECTATIONS FOR DISTANT RECURRENCE IN PATIENTS AT RISK OF METASTATIC DISEASE13
At 5 years, TAFINLAR + MEKINIST reduced the risk of developing metastatic disease in stage III melanoma (per AJCC 8 criteria) vs placebo13
AJCC, American Joint Committee on Cancer; DMFS, distant metastasis-free survival; HR, hazard ratio.
46% REDUCTION IN THE RISK OF DISTANT METASTASIS OR DEATH WITH TAFINLAR + MEKINIST vs placebo in patients with stage IIIC disease13
Stage IIIA: HR, 1.24 (95% CI, 0.42-3.63) for TAFINLAR + MEKINIST (n=50) vs placebo (n=39)13
Stage IIID: HR, 0.20 (95% CI, 0.07-0.55) for TAFINLAR + MEKINIST (n=22) vs placebo (n=17)13
Results at 48 and 60 months were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
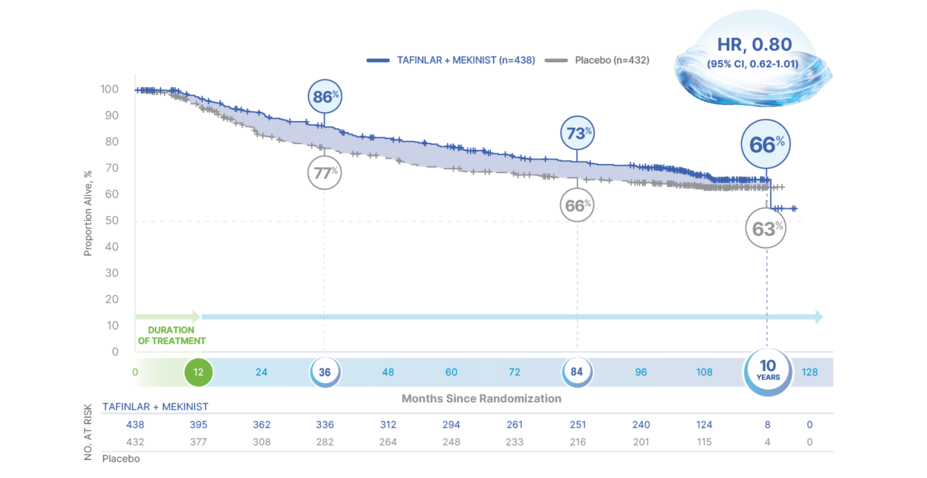
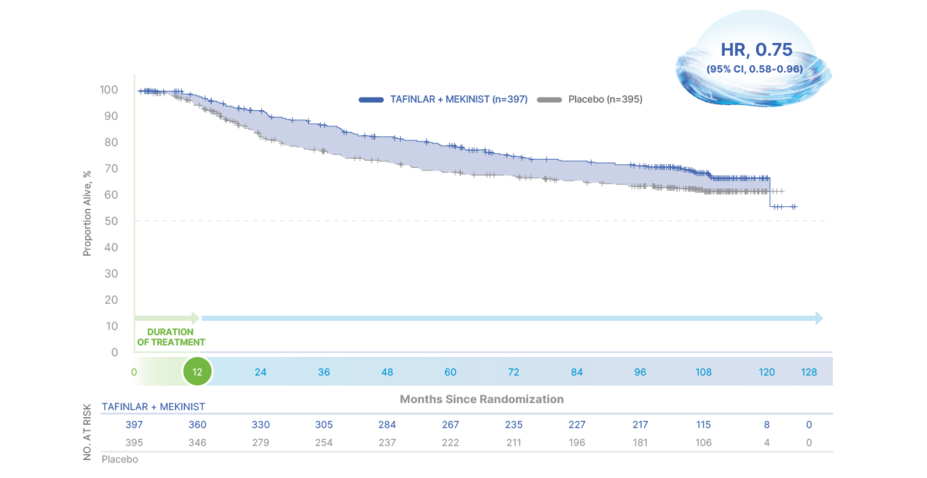
10-Year Overall Survival
TAFINLAR + MEKINIST: THE ONLY ADJUVANT TREATMENT WITH 10 YEARS OF SURVIVAL DATA1,2,9,14-19
66% OVERALL SURVIVAL AT 10 YEARS WITH TAFINLAR + MEKINIST vs 63% WITH PLACEBO (HR, 0.80; 95% CI, 0.62-1.01)9
OS in final analysis of COMBI-ADa
HR, hazard ratio; OS, overall survival; RFS, relapse-free survival.
aData as of July 31, 2023. First interim analysis of OS was taken at the same time as the primary analysis of RFS with a median follow-up of 2.8 years.1,2,9
Median OS was not reached in either treatment arm9
In the TAFINLAR + MEKINIST arm, 37% of patients received subsequent systemic anticancer therapy: 6% received chemotherapy, 29% received immunotherapy, and 21% received small-molecule targeted therapy9
In the control arm, 49% of patients received subsequent systemic anticancer therapy: 7% received chemotherapy, 29% received immunotherapy, and 37% received small-molecule targeted therapy9
Based on 261 events (125 [29%] in the TAFINLAR + MEKINIST arm and 136 [31%] in the placebo arm)9
Results at 3 years, 7 years, and 10 years were not prespecified and are observational in nature; as such, there was no prespecified statistical procedure controlling for type 1 error
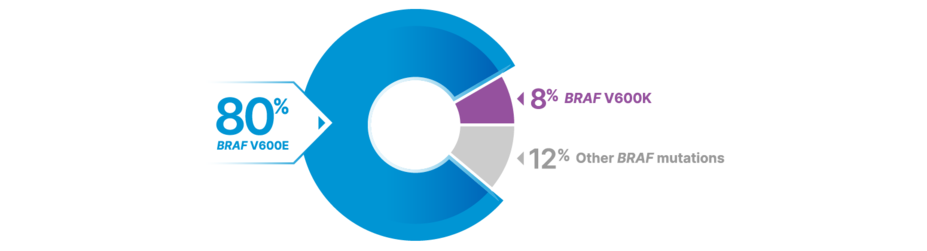
BRAF V600E/K Prevalence
MORE THAN 1 IN 2 PATIENTS WITH MELANOMA MAY HAVE BRAF MUTATIONS—MOST COMMONLY BRAF V600E20
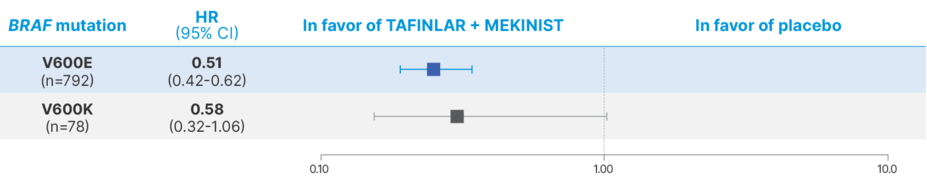
LONG-TERM RESULTS IN THE BRAF V600E/K POPULATIONS
RFS at 5 years in the BRAF V600E/K populations7
HR, hazard ratio.
No formal statistical testing was planned for these subgroup analyses; therefore, no conclusions can be made
OS at 10 years in the BRAF V600E population9
HR, hazard ratio.
In the BRAF V600K subgroup (n=78), the HR for OS at 10 years was 1.95 (95% CI, 0.84-4.50)9
The BRAF V600E and V600K subgroups were predefined, but OS results in these subgroups are observational in nature
No formal statistical testing was planned for these subgroup analyses; therefore, no conclusions can be made
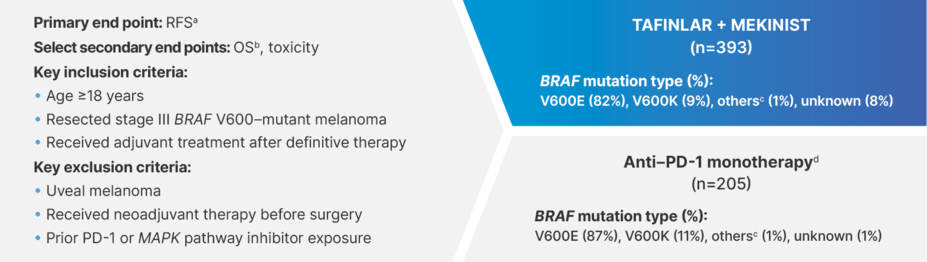
Real-World Evidence
In patients with fully resected stage III BRAF V600–mutant melanoma,
A REAL-WORLD STUDY OF TAFINLAR + MEKINIST vs ANTI–PD-1 MONOTHERAPY21
A retrospective, multicenter cohort study (N=598)21
OS, overall survival; PD-1, programmed cell death protein 1; RFS, relapse-free survival.
aRFS was defined as the period between the definitive surgery and the development of local recurrence and/or distant metastasis, death, or last follow-up, whichever occurred first.21
bOS was defined as the period between the definitive surgery and death or last follow-up, whichever occurred first.21
cIncluded 5 patients with V600R mutations, 1 patient with a V600D mutation, 1 patient with a V600M mutation, and 1 patient with a V600Q mutation across both treatment arms.21
dAnti–PD-1 monotherapies included pembrolizumab, nivolumab, and toripalimab.21
Patients were treated between July 2015 and October 2022 at 1 of 15 melanoma centers in Australia, China, Germany, Italy, Japan, the United Kingdom, and the United States21
Most patient characteristics were well balanced across treatment arms, with the exception of BRAF mutation status (primarily due to different test methods between institutes), type of surgery, and postsurgery radiotherapy21
LIMITATIONS
Although this real-world study had a large sample size, the results from this study need to be interpreted with caution due to its retrospective and nonrandomized nature. A large, prospective, randomized, controlled trial should be performed to confirm the results of this study.21
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations, including a lesser ability to control for confounding factors. Potential confounding factors included: selection bias, given differences in baseline characteristics; lack of randomization; differing mutation-testing methods; imbalanced surgery types and radiotherapy; potential violation of the proportional hazards assumption; lack of tracking for distant metastases after local recurrence; limited follow-up (median: 33 months); and limited ability to provide long-term outcomes (>5 years) for these patients.21
Efficacy outcomes in the overall population21
Median RFS was 51.0 months (95% CI, 41.0-NR) with TAFINLAR + MEKINIST vs 44.8 months (95% CI, 28.5-NR) with anti–PD-1 monotherapy (HR, 0.66; 95% CI, 0.50-0.87)
HR for OS was 1.0 (95% CI, 0.65-1.55)
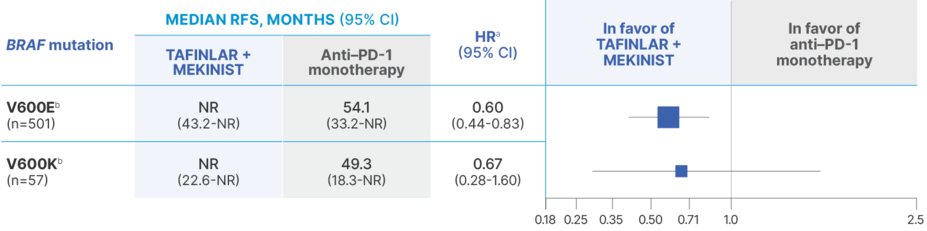
Median RFS outcomes in BRAF V600E/K subgroups21
HR, hazard ratio; NR, not reached; PD-1, programmed cell death protein-1; RFS, relapse-free survival.
aThe exact HR values reported here should be interpreted cautiously due to the concern of violation of the proportional hazards assumption.21
bPreplanned subgroup analyses of the primary end point, RFS, were performed and included BRAF V600 mutation types (V600E and V600K). Subgroup analyses were performed based on complete case analysis and were not adjusted for multiple comparison, thus considered to be exploratory.21
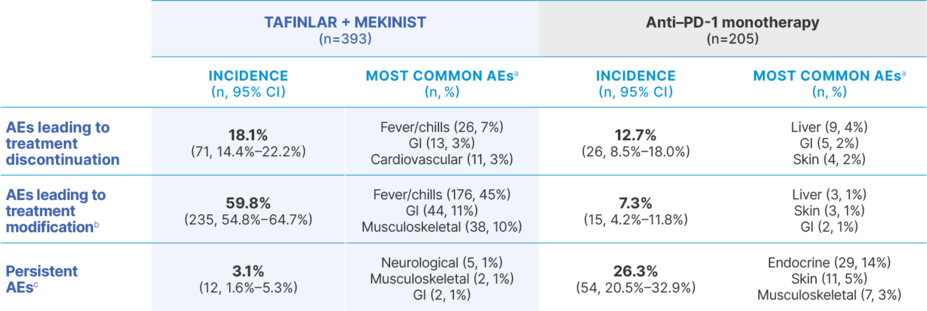
AEs with TAFINLAR + MEKINIST vs anti–PD-1 monotherapy21,22
AE, adverse event; GI, gastrointestinal; PD-1, programmed cell death protein-1.
aSpecific AEs were not defined by the study authors. The 3 most common AEs for each category are listed here. In the TAFINLAR + MEKINIST cohort, additional AEs leading to treatment modification included skin (n=33, 8%) and liver (n=31, 8%) toxicities, and additional AEs leading to treatment discontinuation included liver toxicities (n=10, 3%). In the anti–PD-1 monotherapy cohort, additional AEs leading to treatment modification or discontinuation included immune-related hepatitis (n=9, 4%).21
bIncluding both dose reduction (TAFINLAR + MEKINIST only) and treatment interruption (both TAFINLAR + MEKINIST and anti–PD-1 monotherapy).21
cPersistent AEs were defined as those that remained present >3 months following treatment discontinuation.21