Dosing & Administration
Recommended Dose
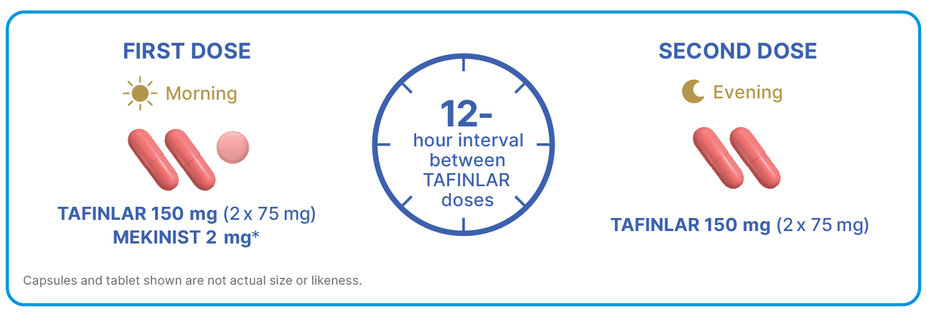
CONVENIENT AT-HOME DOSING: 3 PILLS IN THE MORNING AND 2 AT NIGHT
Recommended dosing: TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily1,2
*The recommended dosage for MEKINIST is 2 mg taken at the same time each day, approximately 24 hours apart. Time of day is based on personal preference and is represented in this piece as morning for presentation purposes only.
Do not take a missed dose of TAFINLAR within 6 hours of the next dose, or a missed dose of MEKINIST within 12 hours of the next dose1,2
Do not open, crush, or break TAFINLAR capsules1
Keep TAFINLAR, MEKINIST, and all medicine out of the reach of children
Treatment with TAFINLAR + MEKINIST should continue until disease progression or unacceptable toxicity occurs1,2
Dose Modifications
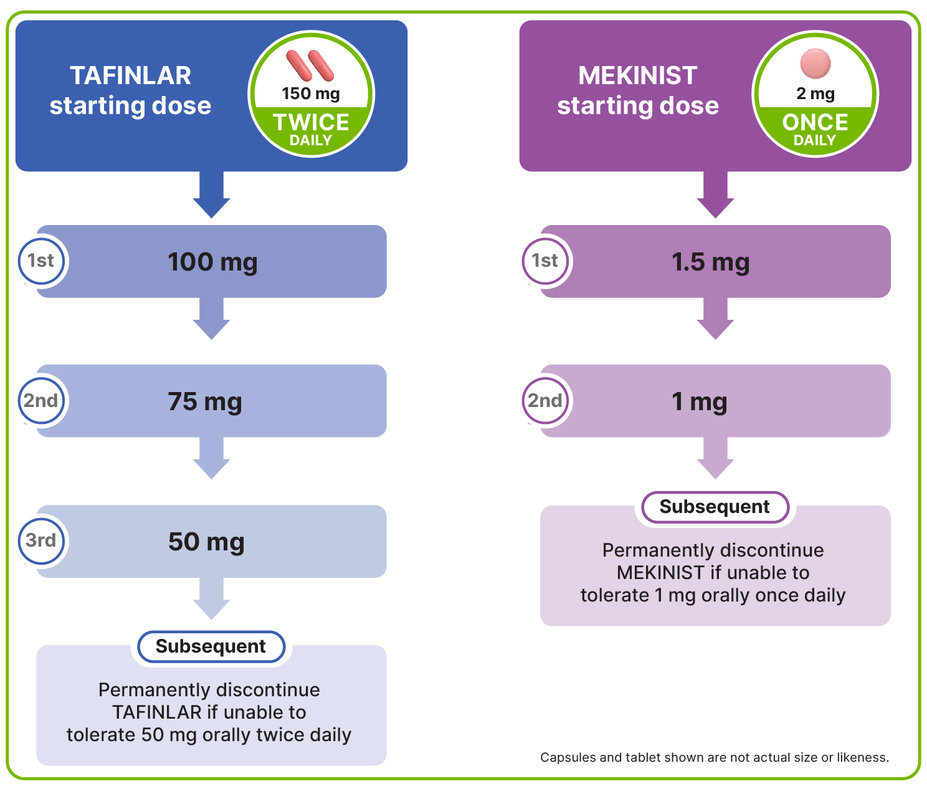
DOSE MODIFICATION, INTERRUPTION, OR DISCONTINUATION MAY BE REQUIRED TO MANAGE CERTAIN ADVERSE REACTIONS
Dose reductions for TAFINLAR + MEKINIST1,2
To aid in dose reductions, TAFINLAR is also available in 50-mg capsules and MEKINIST is also available in 0.5-mg tablets1,2
TAFINLAR + MEKINIST: In the NSCLC clinical trial, the most commonly occurring adverse reactions (≥20%) in patients receiving the combination were pyrexia (55%), fatigue (51%), nausea (45%), vomiting (33%), diarrhea (32%), dry skin (31%), decreased appetite (29%), edema (28%), rash (28%), chills (23%), hemorrhage (23%), cough (22%), and dyspnea (20%)1,2
Dose modification, interruption, or discontinuation may be required to manage certain adverse reactions. Please see full TAFINLAR Prescribing Information and full MEKINIST Prescribing Information for details1,2
DRUG INTERACTIONS
Avoid concurrent administration of strong inhibitors of CYP3A4 or CYP2C8
Avoid concurrent administration of strong inducers of CYP3A4 or CYP2C8
Concomitant use with agents that are sensitive substrates of CYP3A4, CYP2C8, CYP2C9, CYP2C19, or CYP2B6 may result in loss of efficacy of these agents
Storage and Handling
TAFINLAR capsules should be stored at room temperature at 68 °F to 77 °F (20 °C-25 °C); excursions permitted between 59 °F and 86 °F (15 °C-30 °C). Store and dispense in the original bottle with the desiccant1
MEKINIST tablets should be stored at room temperature between 68 °F to 77 °F (20 °C-25 °C); excursions permitted between 59 °F and 86 °F (15 °C-30 °C). Dispense in original bottle. Do not remove desiccant. Protect from moisture and light. Do not place medication in pill boxes2