Efficacy
Study Design of Clinical Trial
TAFINLAR + MEKINIST: A PROVEN TARGETED THERAPY FOR PATIENTS WITH BRAF V600E METASTATIC NSCLC
The safety and efficacy of TAFINLAR in combination with MEKINIST were evaluated in a multi-center, 3-cohort, nonrandomized, activity-estimating, open-label trial (Study BRF113928, NCT01336634).1,2
Key eligibility criteria were locally confirmed BRAF V600E mutation–positive metastatic NSCLC, no prior exposure to BRAF or MEK inhibitors, and absence of EGFR mutation or ALK rearrangement (unless patients had progression on prior tyrosine kinase inhibitor therapy).1,2
FIRST-LINE PATIENTS | PREVIOUSLY TREATED PATIENTS | |
Patients with BRAF V600E mutation–positive NSCLC who have not received prior systemic treatment | Patients with BRAF V600E mutation–positive NSCLC who have progressed on 1 to 3 lines of platinum-containing chemotherapy | Patients with BRAF V600E mutation–positive NSCLC who have progressed on 1 to 3 lines of platinum-containing chemotherapy |
n=36 TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily (Cohort C) | n=57 TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily (Cohort B) | n=78 TAFINLAR 150 mg twice daily (Cohort A) |
Major efficacy outcomes studied: ORR and DOR
Additional outcomes: OS, PFS, and safety1-3,*
ALK, anaplastic lymphoma kinase; DOR, duration of response; EGFR, epithelial growth factor receptor; MEK, mitogen-activated extracellular signal-regulated kinase; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors.
*The major efficacy outcomes were ORR per Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1) as assessed by independent review committee and DOR.1,2
First-Line Patients: Response Rates
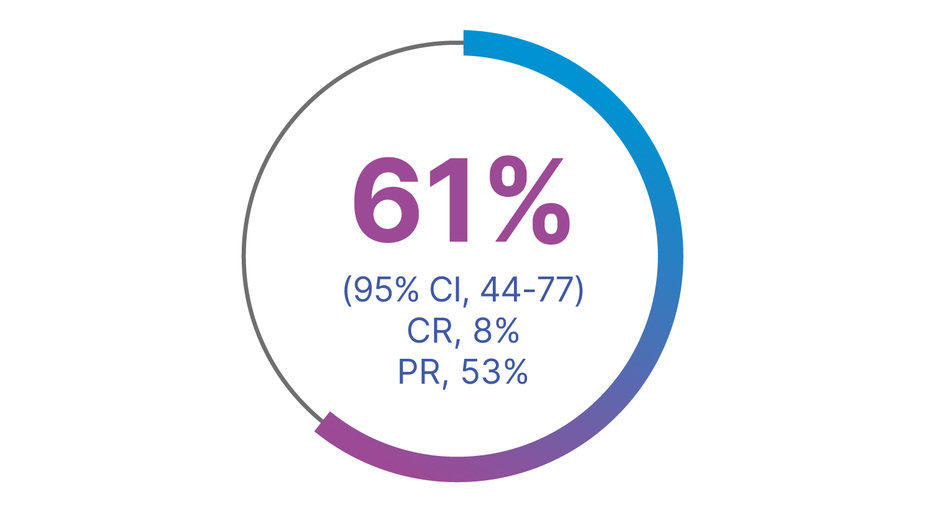
PROVEN RESPONSE RATES IN FIRST-LINE PATIENTS (n=36) WITH TAFINLAR + MEKINIST1,2
Overall response rate1,2
CR, complete response; PR, partial response.
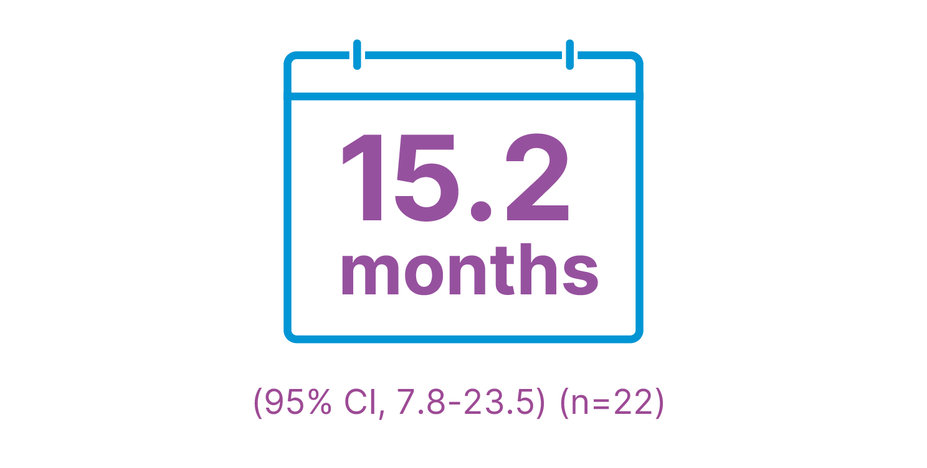
Median duration of response1,2
Efficacy in Previously Treated Patients: Response Rates
PROVEN RESPONSE RATES FOR TAFINLAR + MEKINIST IN PREVIOUSLY TREATED PATIENTS
Based on independent review (n=57)1,2
Overall response rate: 61% (95% CI, 48-74) (CR, 5%; PR, 56%)
Median DOR: 9 months (95% CI, 5.8-26.2) (n=35)
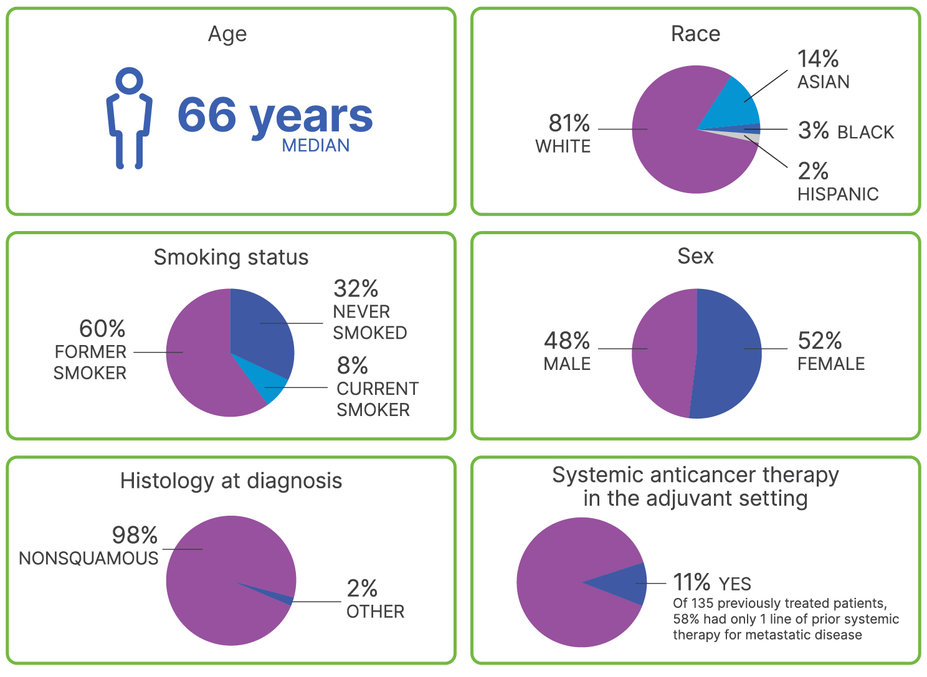
Patient Characteristics
PROFILE OF PATIENTS IN TAFINLAR + MEKINIST NSCLC STUDY
Patient characteristics across all 3 cohorts1,2