Safety Profile
Adverse Reactions
A WELL-ESTABLISHED SAFETY PROFILE
Adverse reactions occurring in ≥20% (all grades) of patients treated with TAFINLAR + MEKINIST1,2,a
TAFINLAR + MEKINIST (N=93) | ||
Adverse reactions | All grades, % | Grades 3 and 4, % |
General | ||
Pyrexia | 55 | 5 |
Fatigueb | 51 | 5 |
Edemac | 28 | 0 |
Chills | 23 | 1.1 |
Gastrointestinal | ||
Nausea | 45 | 0 |
Vomiting | 33 | 3.2 |
Diarrhea | 32 | 2.2 |
Decreased appetite | 29 | 0 |
Skin | ||
Dry skin | 31 | 1.1 |
Rashd | 28 | 3.2 |
Vascular | ||
Hemorrhagee | 23 | 3.2 |
Respiratory system | ||
Cough | 22 | 0 |
Dyspnea | 20 | 5 |
aNational Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
bIncludes fatigue, malaise, and asthenia.
cIncludes peripheral edema, edema, and generalized edema.
dIncludes rash, rash generalized, rash papular, rash macular, rash maculopapular, and rash pustular.
eIncludes hemoptysis, hematoma, epistaxis, purpura, hematuria, subarachnoid hemorrhage, gastric hemorrhage, urinary bladder hemorrhage, contusion, hematochezia, injection site hemorrhage, pulmonary hemorrhage, and retroperitoneal hemorrhage.
Other clinically important adverse reactions for TAFINLAR observed in <10% of patients with NSCLC receiving TAFINLAR administered with MEKINIST were:
Gastrointestinal: Pancreatitis
Renal and Urinary: Tubulointerstitial nephritis
Treatment-Emergent Laboratory Abnormalities
Treatment-emergent laboratory abnormalities occurring at ≥20% (all grades) of patients receiving TAFINLAR + MEKINIST1,2
TAFINLAR + MEKINIST (N=93) | ||
All grades, % | Grades 3 and 4, % | |
| Chemistrya | ||
| Hyperglycemia | 71 | 9 |
| Hyponatremia | 57 | 17 |
| Hypophosphatemia | 36 | 7 |
| Increased creatinine | 21 | 1.1 |
| Hepatica | ||
| Increased blood alkaline phosphatase | 64 | 0 |
| Increased AST | 61 | 4.4 |
| Increased ALT | 32 | 6 |
| Hematologyb | ||
| Leukopenia | 48 | 8 |
| Anemia | 46 | 10 |
| Neutropenia | 44 | 8 |
| Lymphopenia | 42 | 14 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
aFor these laboratory tests the denominator is 90.
bFor these laboratory tests the denominator is 91.
Discontinuation Rates
PERCENTAGES OF PATIENTS WHOSE DOSE OF TAFINLAR + MEKINIST WAS DISCONTINUED, REDUCED, OR INTERRUPTED DUE TO ADVERSE REACTIONS
TAFINLAR
18% TAFINLAR discontinuation rate due to adverse reactions; the most common were pyrexia (2.2%), ejection fraction decreased (2.2%), and respiratory distress (2.2%)1
Adverse reactions leading to dose reductions of TAFINLAR occurred in 35% of patients receiving TAFINLAR + MEKINIST; the most common were pyrexia (10%), diarrhea (4.3%), nausea (4.3%), vomiting (4.3%), and neutropenia (3.2%)1
Adverse reactions leading to dose interruptions of TAFINLAR occurred in 62% of patients; the most common were pyrexia (27%), vomiting (11%), neutropenia (8%), and chills (6%)1
MEKINIST
19% MEKINIST discontinuation rate due to adverse reactions; the most common were pyrexia (2.2%), ejection fraction decreased (2.2%), and respiratory distress (2.2%)2
Adverse reactions leading to dose reductions of MEKINIST occurred in 30% of patients receiving MEKINIST + TAFINLAR; the most common were pyrexia (5%), nausea (4.3%), vomiting (4.3%), diarrhea (3.2%), and neutropenia (3.2%)2
Adverse reactions leading to dose interruptions of MEKINIST occurred in 57% of patients receiving MEKINIST + TAFINLAR; the most common were pyrexia (16%), vomiting (10%), neutropenia (8%), nausea (5%), and ejection fraction decreased (5%)2
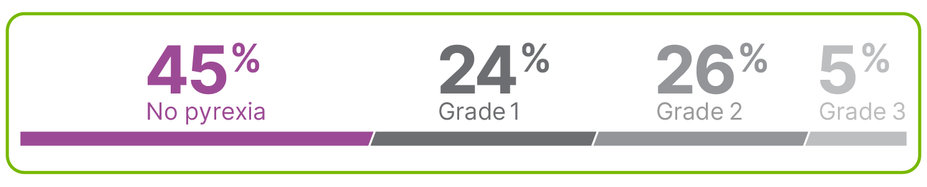
Incidence of Pyrexia
PYREXIA WAS ONE OF THE MOST COMMON ADVERSE REACTIONS WITH TAFINLAR + MEKINIST, LEADING TO DISCONTINUATION IN 2.2% OF PATIENTS1,2
Incidence of pyrexia in the NSCLC study (N=93)3
55% of patients experienced pyrexia
Pyrexia occurred in patients taking TAFINLAR + MEKINIST in the NSCLC study
No patients experienced grade 4 pyrexia
2.2% of patients discontinued TAFINLAR + MEKINIST due to pyrexia
In patients receiving the combination who experienced fever3,4
Grade 1: Temperature of 100.4 °F to 102.2 °F
Grade 2: Temperature of 102.3 °F to 104 °F
Grade 3: Temperature of >104 °F for ≤24 hours
Grade 4: Temperature of >104 °F for >24 hours
PATIENT COUNSELING
Inform patients that fever is common during treatment with TAFINLAR + MEKINIST but may also be serious. When taking TAFINLAR + MEKINIST, fever may happen more often or may be more severe. In some cases, chills or shaking chills, too much fluid loss (dehydration), low blood pressure, dizziness, or kidney problems may happen with the fever
Advise patients to tell their health care provider right away if they get a fever during treatment with TAFINLAR + MEKINIST
DON'T LET PYREXIA GET IN THE WAY OF YOUR PATIENT'S TREATMENT
In patients treated with combination therapy across clinical studies, fever occurred in 58% of patients receiving TAFINLAR with MEKINIST. Serious febrile reactions and fever of any severity complicated by hypotension, rigors or chills, dehydration or renal failure occurred in 5% of patients. Fever was complicated by hypotension in 4%, dehydration in 3%, syncope in 2%, renal failure in 1%, and severe chills/rigors in <1% of patients.1,2
THREE SIMPLE STEPS TOWARD PYREXIA MANAGEMENT1,2,a
PYREXIA (≥100.4 °F) | 1 | InterruptTAFINLAR + MEKINIST at the onset of pyrexia (≥100.4 °F) OR first symptom in case of recurrence. | |||||||||
2 | Manage
| ||||||||||
3 | Restart
| ||||||||||
| Manage pyrexia with antipyretics or corticosteroids, depending on episode and severity | |||||||||||
Antipyretics can be used to help manage pyrexia, including as secondary prophylaxis when resuming TAFINLAR + MEKINIST if patient had a prior episode of severe febrile reaction or fever associated with complications. | Corticosteroids can be considered and administered for at least 5 days for second or subsequent pyrexia if temperature doesn't return to baseline within 3 days of onset of pyrexia, or for pyrexia associated with complications, such as dehydration, hypotension, renal failure, or severe chills/rigors, and there is no evidence of active infection. | Monitor renal function and serum creatinine during and following severe pyrexia. | |||||||||
aSee the Prescribing Information for TAFINLAR and Prescribing Information for MEKINIST for additional details.
Recommended Dose Modifications for Adverse Reactions
Severity of Adverse Reactiona | TAFINLAR1,b | MEKINIST2,b |
Hemorrhage | ||
| Withhold TAFINLAR
| Withhold MEKINIST
|
| Permanently discontinue TAFINLAR | Permanently discontinue MEKINIST |
New Primary Malignancies | ||
| Permanently discontinue TAFINLAR | No dose modifications recommended for MEKINIST |
| Venous Thromboembolism | ||
| No dose modifications recommended for TAFINLAR | Withhold MEKINIST for up to 3 weeks
|
| Dose modifications are not included in TAFINLAR Prescribing Information | Permanently discontinue MEKINIST |
Cardiomyopathy | ||
| Dose modifications are not included in TAFINLAR Prescribing Information | Withhold MEKINIST for up to 4 weeks
|
| Withhold TAFINLAR until LVEF improves to at least the institutional LLN and absolute decrease to less than or equal to 10% compared to baseline, then resume at same dose | Permanently discontinue MEKINIST |
Ocular Toxicities | ||
| No dose modifications recommended for TAFINLAR | Withhold MEKINIST for up to 3 weeks
|
| No dose modifications recommended for TAFINLAR | Permanently discontinue MEKINIST |
| Withhold TAFINLAR for up to 6 weeks
| No dose modifications recommended for MEKINIST |
Pulmonary | ||
| No dose modifications recommended for TAFINLAR | Permanently discontinue MEKINIST |
Febrile Reactions | ||
| Withhold TAFINLAR and MEKINIST until fever resolves, then resume TAFINLAR and MEKINIST at same or lower dose | |
|
Or
| |
Skin Toxicities | ||
| Withhold TAFINLAR and MEKINIST for up to 3 weeks
| |
| Permanently discontinue TAFINLAR and MEKINIST | |
Other Adverse Reactions | ||
| Withhold TAFINLAR and MEKINIST
| |
|
Or
| |
|
| |
DVT, deep vein thrombosis; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; PE, pulmonary embolism; RAS, monomeric G protein.
aNational Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0.
bSee recommended dose reductions of TAFINLAR and MEKINIST under Dosing and Administration tab.